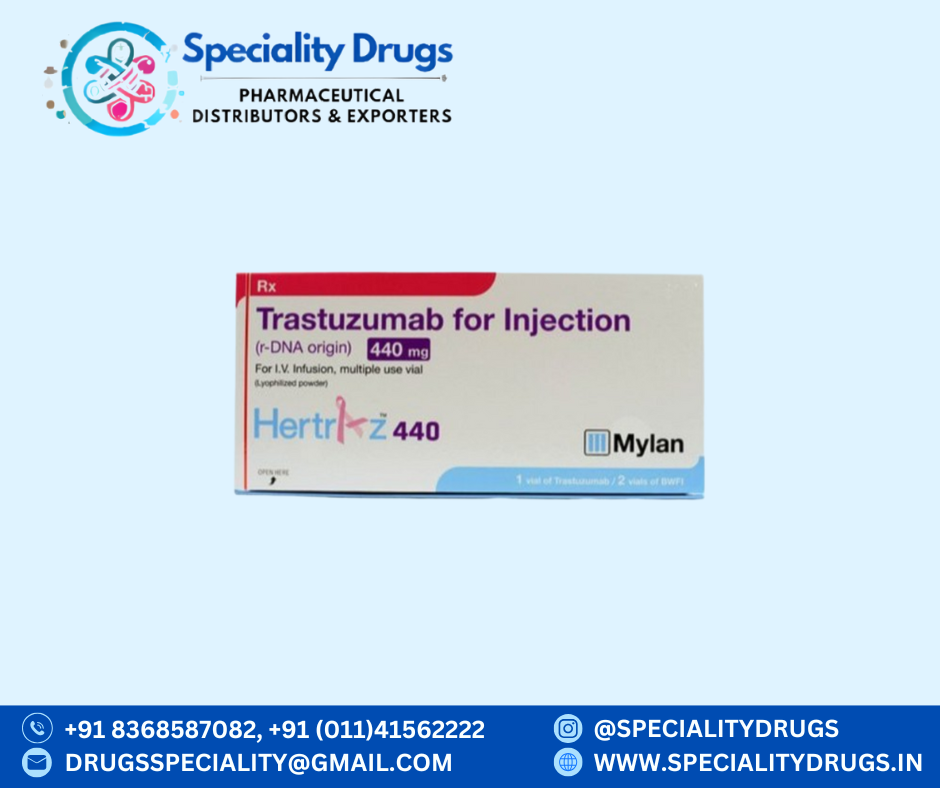
What is HERTRAZ 440MG?
HERTRAZ 440MG is an anti-cancer injection containing Trastuzumab as its active ingredient. It is a monoclonal antibody that targets and binds to the HER2 (human epidermal growth factor receptor 2) protein, which is overexpressed in certain types of breast and stomach cancers.
By blocking the HER2 receptor, HERTRAZ 440MG helps inhibit the growth and spread of cancer cells.
What is the Use of HERTRAZ 440MG?
HERTRAZ 440MG is prescribed for:
-
HER2-positive breast cancer (early-stage and metastatic)
-
HER2-positive gastric (stomach) or gastroesophageal junction cancer
It may be used alone or in combination with other chemotherapy drugs such as paclitaxel, docetaxel, or cisplatin.
Benefits of HERTRAZ 440MG
-
Provides targeted therapy specifically for HER2-positive cancers
-
Improves survival and reduces recurrence rates in breast cancer patients
-
Slows down tumor growth and metastasis
-
Can be safely combined with standard chemotherapy for enhanced effectiveness
How HERTRAZ 440MG Works
HERTRAZ 440MG (Trastuzumab) binds to HER2 receptors on the surface of cancer cells, blocking the growth-promoting signals and activating the immune system to destroy these cancer cells.
Side Effects of HERTRAZ 440MG
Common Side Effects:
-
Fever or chills
-
Nausea and vomiting
-
Headache and fatigue
-
Diarrhea
-
Rash or itching
Serious Side Effects (require medical attention):
-
Heart problems (heart failure or irregular heartbeat)
-
Shortness of breath or chest pain
-
Severe allergic reactions
-
Lung or liver complications
Precautions & Warnings
-
Use only under medical supervision by an oncologist.
-
Heart function monitoring is essential before and during treatment.
-
Not recommended during pregnancy or breastfeeding.
-
Inform your doctor of any existing heart or lung conditions.
Storage & Administration
-
Store in a refrigerator (2°C – 8°C).
-
Do not freeze or shake the vial.
-
Administered intravenously (IV) by a qualified healthcare professional.
Product Summary
-
Product Name: HERTRAZ 440MG Injection
-
Salt Name: Trastuzumab
-
Strength: 440 mg
-
Manufacturer: Mylan Pharmaceuticals Pvt. Ltd.
-
Storage: Store at 2°C – 8°C. Do not freeze or shake.
-
Presentation: Single-use vial for intravenous infusion
1. What is HERTRAZ 440MG used for?
It is used for treating HER2-positive breast and gastric cancers.
2. What is the active ingredient in HERTRAZ 440MG?
The active ingredient is Trastuzumab.
3. How does HERTRAZ 440MG work?
It binds to HER2 receptors on cancer cells, blocking growth signals and triggering immune destruction.
4. How is HERTRAZ 440MG administered?
It is given through intravenous infusion under medical supervision.
5. How often is HERTRAZ 440MG given?
Usually every 1 to 3 weeks, depending on the treatment protocol.
6. Can HERTRAZ 440MG be used alone?
Yes, or in combination with chemotherapy, based on cancer type and stage.
7. What are the common side effects?
Fever, chills, fatigue, nausea, diarrhea, and headache.
8. Can HERTRAZ 440MG cause heart problems?
Yes, it may affect heart function; monitoring is required during therapy.
9. Is HERTRAZ 440MG safe during pregnancy?
No, it should not be used during pregnancy or breastfeeding.
10. How long is the treatment duration?
It varies, typically from 6 months to 1 year, depending on response.
11. Can HERTRAZ 440MG be taken with other drugs?
Yes, but only under oncologist supervision to avoid interactions.
12. How should HERTRAZ 440MG be stored?
Refrigerate at 2°C–8°C. Do not freeze or expose to direct light.
13. What should I do if I miss a dose?
Contact your doctor immediately to reschedule your infusion.
14. Who should avoid HERTRAZ 440MG?
Patients with heart failure, severe lung disease, or allergy to Trastuzumab.
15. Who manufactures HERTRAZ 440MG?
It is manufactured by Mylan Pharmaceuticals Pvt. Ltd., a trusted oncology medicine producer.

Reviews
There are no reviews yet.