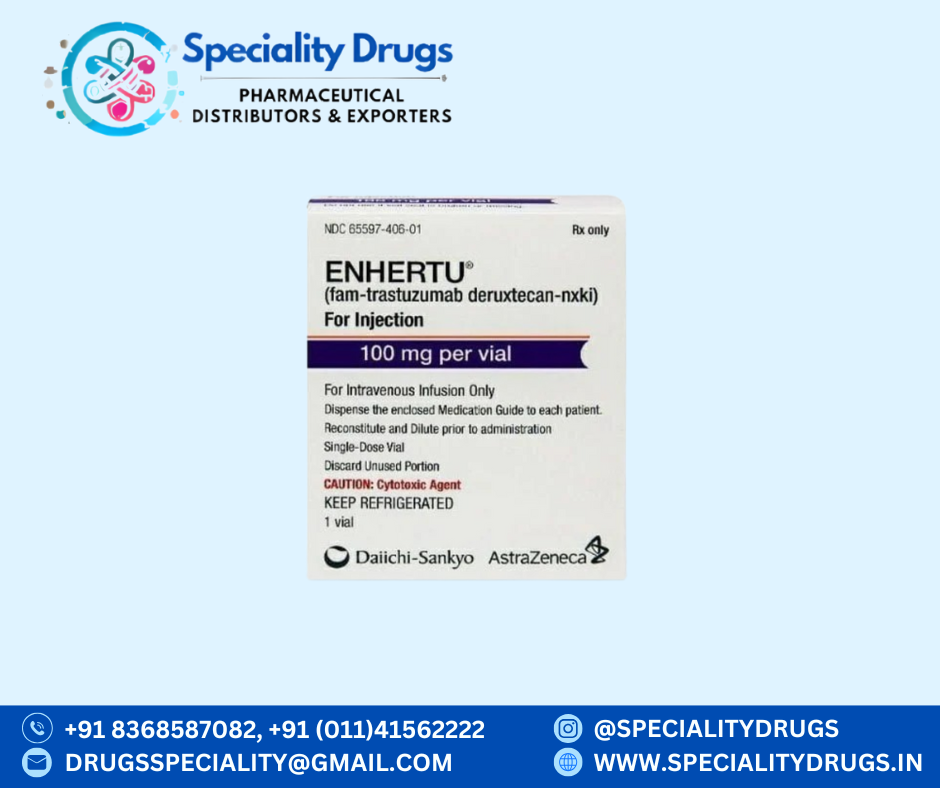
What is ENHERTU 100 mg?
ENHERTU 100 mg (trastuzumab deruxtecan) is a prescription anticancer medicine classified as an antibody–drug conjugate (ADC). It combines a HER2-targeted monoclonal antibody with a potent chemotherapy agent to help destroy cancer cells while limiting damage to healthy tissue.
What is the use of ENHERTU 100 mg?
ENHERTU is used for the treatment of adults with:
-
HER2-positive metastatic breast cancer who have previously received other anti-HER2 therapies.
-
Unresectable or metastatic HER2-positive gastric or gastroesophageal junction adenocarcinoma after prior treatments.
-
HER2-mutant non-small cell lung cancer (NSCLC) in patients who have received prior systemic therapy.
Its use is guided by a specialist after confirming HER2 status through appropriate testing.
Benefits of ENHERTU 100 mg
-
Targets HER2 receptors precisely, delivering chemotherapy directly to cancer cells.
-
Demonstrated significant tumor shrinkage and improved progression-free survival in clinical studies.
-
Offers a treatment option for patients who have limited alternatives after standard therapies fail.
Side Effects of ENHERTU 100 mg
Common side effects may include:
-
Nausea, vomiting, loss of appetite
-
Fatigue, low white blood cell count (neutropenia)
-
Hair loss, cough, or constipation
Serious but less common risks include interstitial lung disease (ILD), decreased heart function, and severe infusion reactions. Patients should be closely monitored by their oncologist, and any new or worsening symptoms should be reported immediately.
1. What is ENHERTU 100 mg and how does it work?
ENHERTU is an antibody–drug conjugate that targets HER2 receptors on cancer cells, delivering chemotherapy directly to destroy them.
2. What types of cancer is ENHERTU used to treat?
It is approved for HER2-positive metastatic breast cancer, HER2-positive gastric/gastroesophageal junction cancer, and HER2-mutant non-small cell lung cancer (NSCLC).
3. Is ENHERTU a chemotherapy or targeted therapy?
It is a combination of both—a targeted HER2 antibody linked to a chemotherapy drug, making it a targeted chemotherapy treatment.
Administration & Dosage
4. How is ENHERTU 100 mg given?
It is administered as an intravenous (IV) infusion by a healthcare professional, usually every 3 weeks or as prescribed by your oncologist.
5. How long does each infusion of ENHERTU take?
The first infusion typically takes about 90 minutes. Subsequent infusions may be given over 30 minutes if well tolerated.
6. How is the dosage of ENHERTU determined?
The dose is based on body weight and clinical condition, as decided by the treating oncologist.
Safety & Side Effects
7. What are the most common side effects of ENHERTU?
Nausea, vomiting, fatigue, hair loss, decreased appetite, low white blood cell count, and cough are common.
8. What serious risks should I watch for while on ENHERTU?
Potentially serious side effects include interstitial lung disease (ILD), decreased heart function, and severe infusion reactions.
9. How often will I need medical monitoring during treatment?
Regular blood tests, heart function evaluations, and lung assessments are necessary to monitor for side effects.
Precautions & Interactions
10. Can ENHERTU be used during pregnancy or breastfeeding?
No. ENHERTU can harm an unborn baby and is not recommended during pregnancy or while breastfeeding.
11. Are there any drug interactions with ENHERTU?
There are no major known drug–drug interactions, but patients should share a complete list of medications with their doctor.
12. What should I do if I miss a scheduled infusion?
Contact your oncologist immediately to reschedule; do not attempt to self-administer or adjust the dose.
Effectiveness & Expectations
13. How soon can I expect to see results with ENHERTU treatment?
Response varies, but clinical studies show tumor shrinkage in many patients after a few treatment cycles.
14. Can ENHERTU cure cancer?
ENHERTU is not a cure but can significantly slow disease progression and improve quality of life.
15. How long can I stay on ENHERTU treatment?
Patients usually continue therapy as long as they respond positively and side effects remain manageable, as decided by their oncologist.


Reviews
There are no reviews yet.